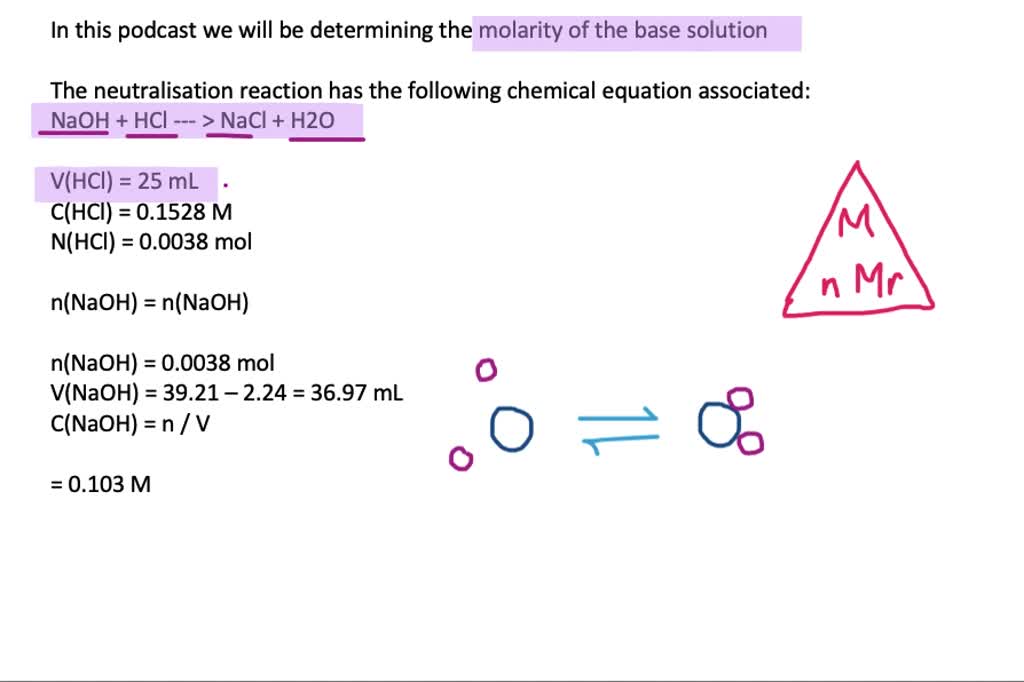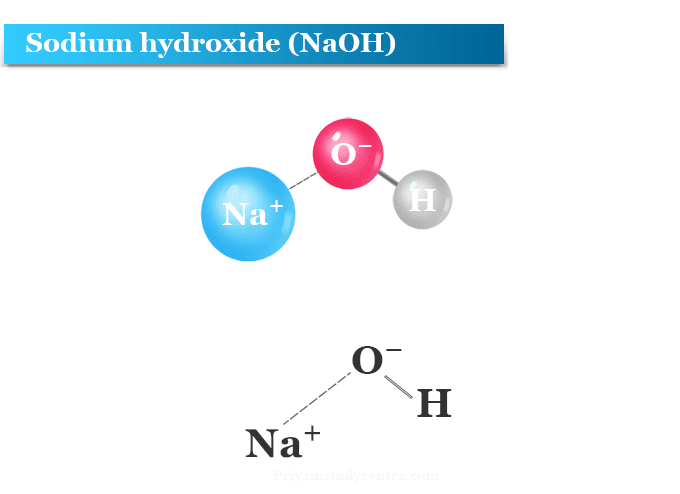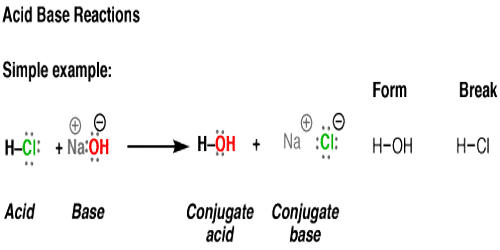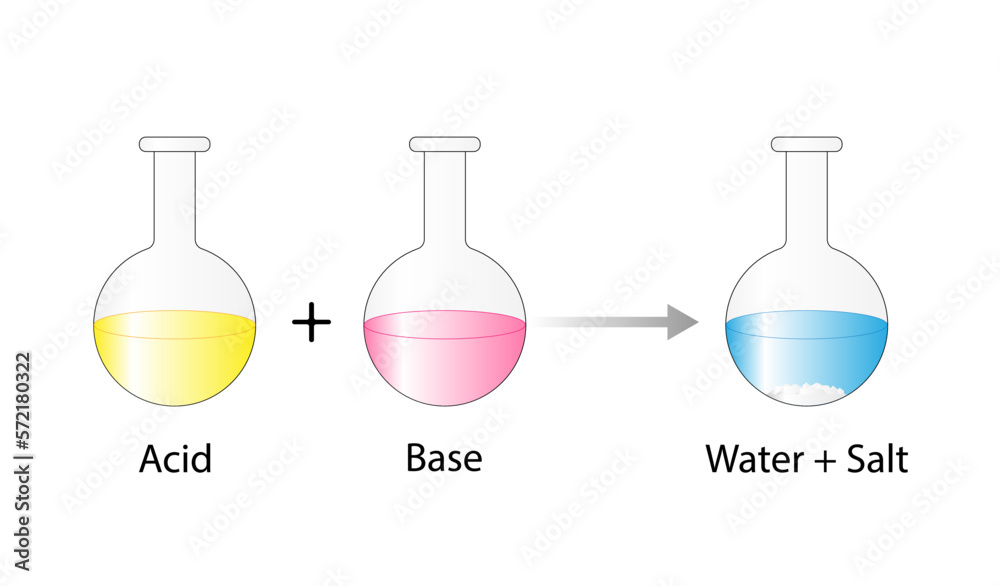
Acid–base reaction. chemical reaction neutralization. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Vector illustration. Stock Vector | Adobe Stock

Acid – base reaction. chemical reaction neutralization the acid and base properties, producing a salt and water. used to determine pH. Bronsted – Lowry theory. molecules of HCl, NaOH, H2O, and NaCl,
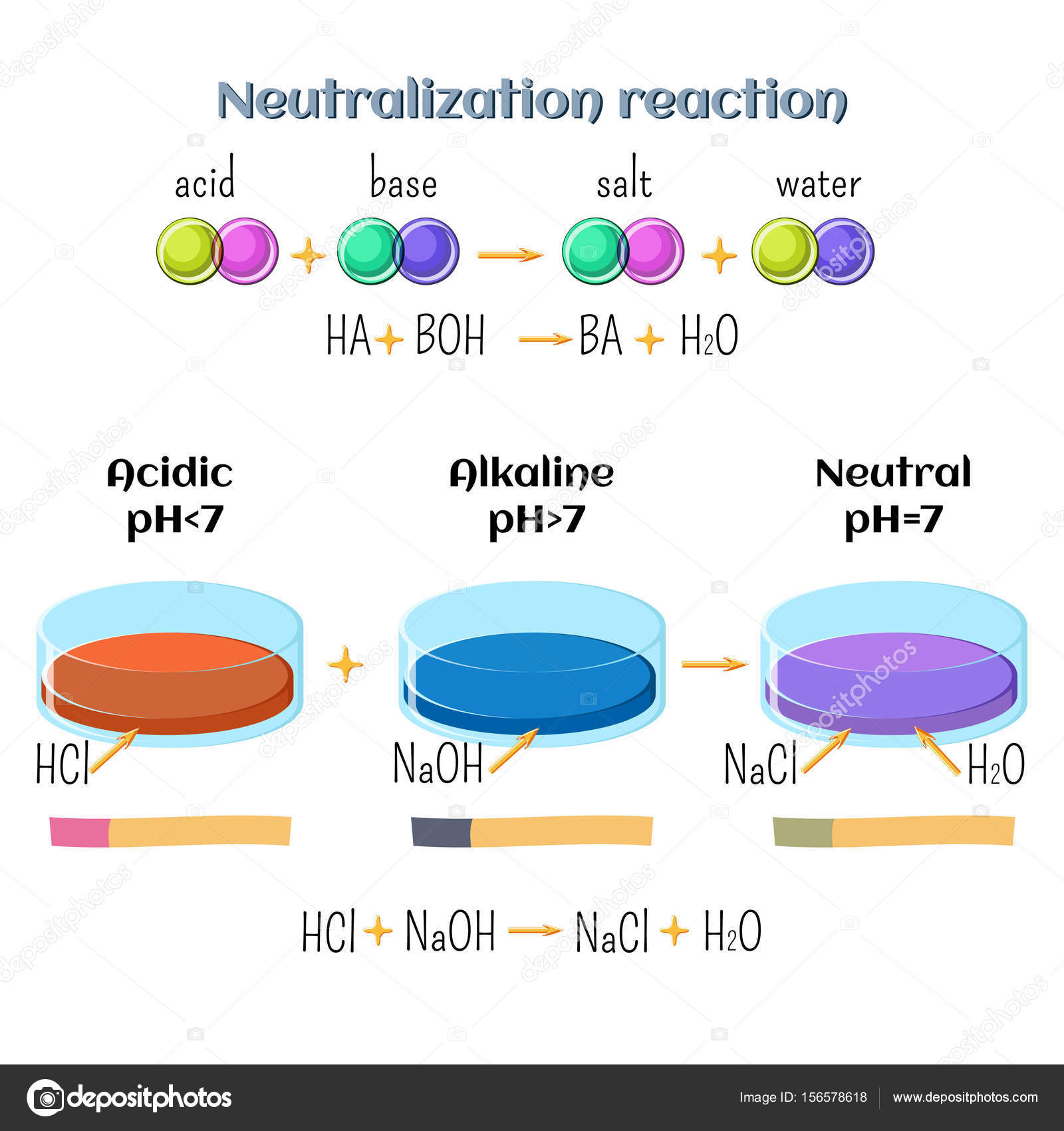
Acid-base, neutralization reaction of hydrochloric acid and sodium hydroxide. Types of chemical reactions, part 6 of 7. Stock Vector Image by ©inkoly #156578618
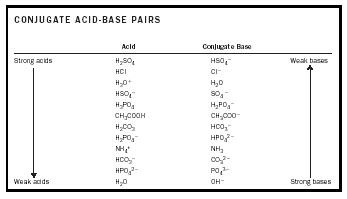
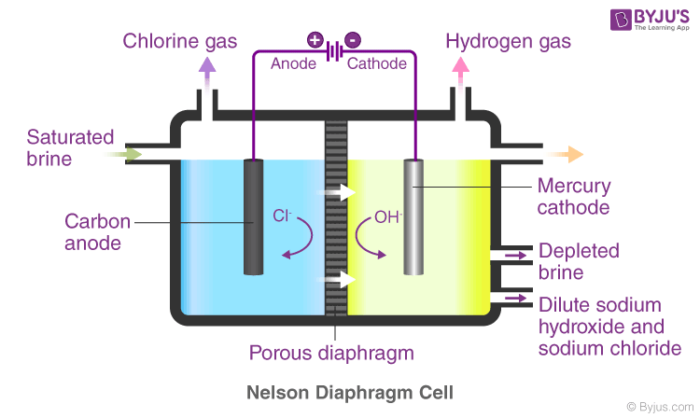
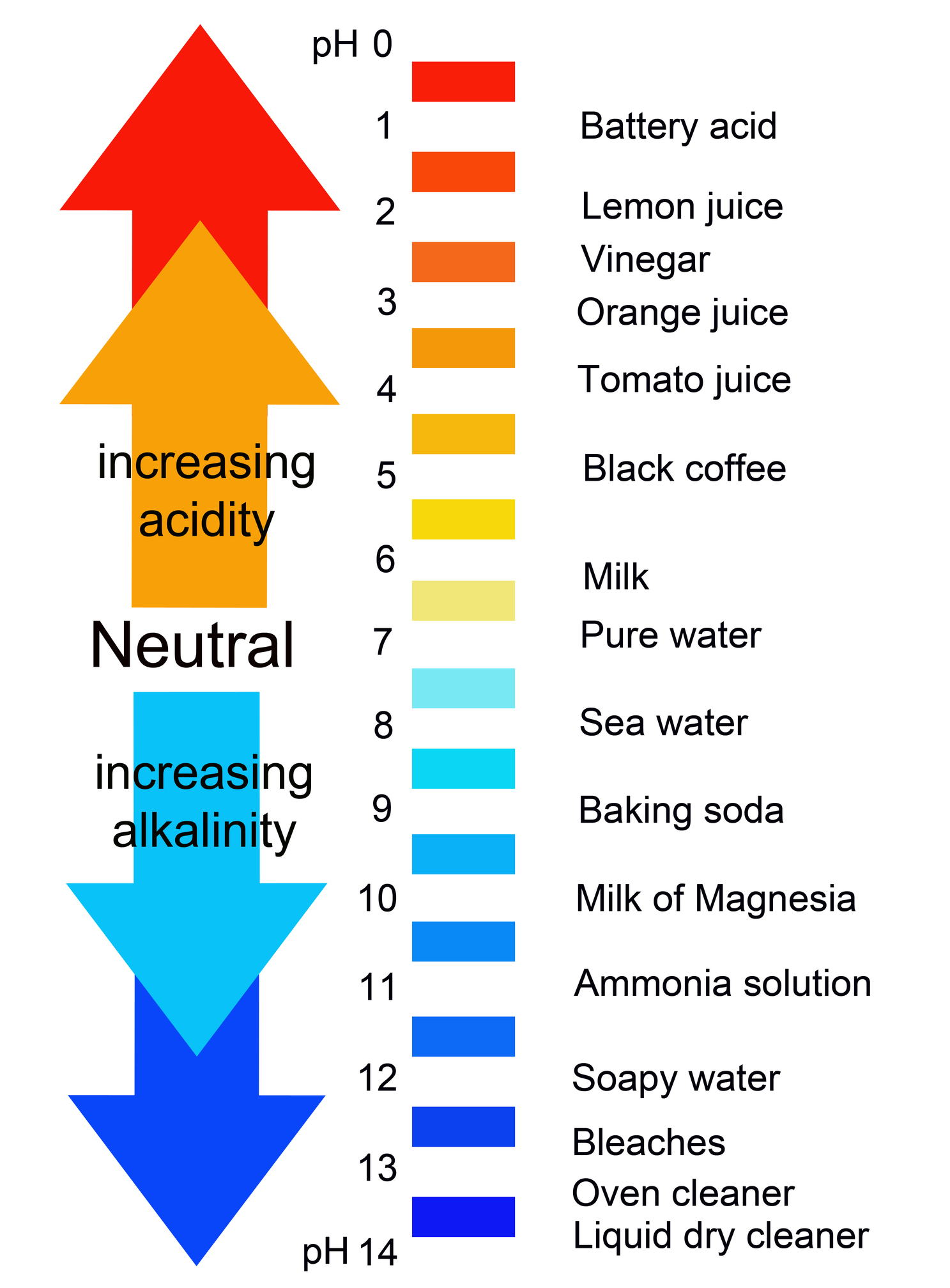
Acid-Base Chemistry - Chemistry Encyclopedia - reaction, water, metal, gas, number, equation, salt, property

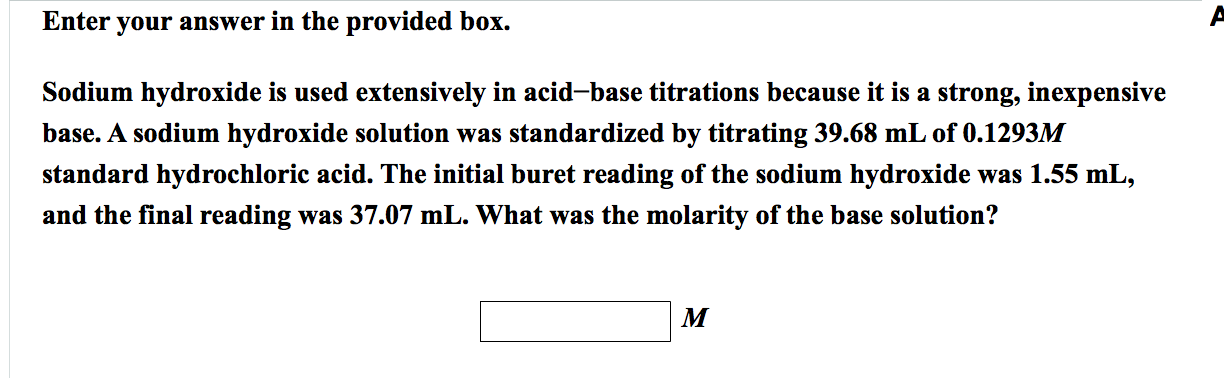
Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and write the equation for this process.
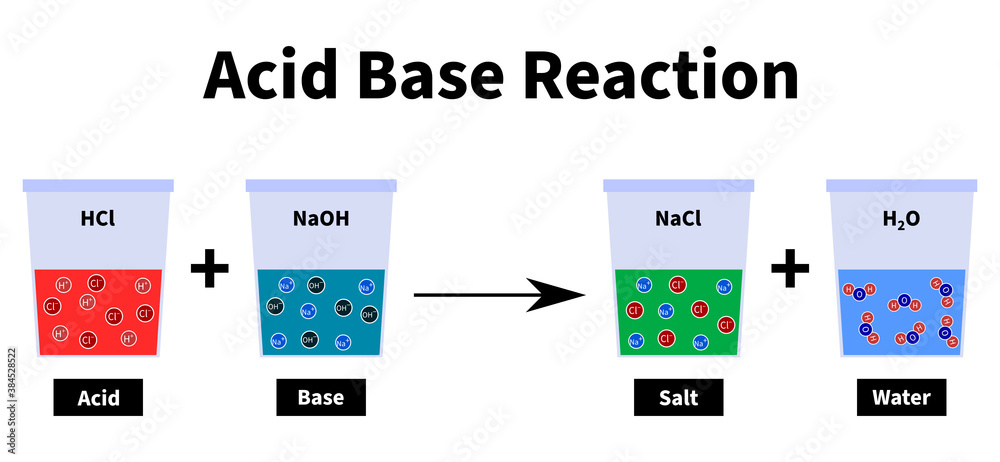
Acid base reaction: salt water hydrogen chloride sodium hydroxide sodium chloride water, neuatralization, chemical reaction Stock Illustration | Adobe Stock

Selective focus of sodium hydroxide base and sulfuric acid solution in brown glass and plastic bottle inside a chemistry laboratory. White background Stock Photo - Alamy