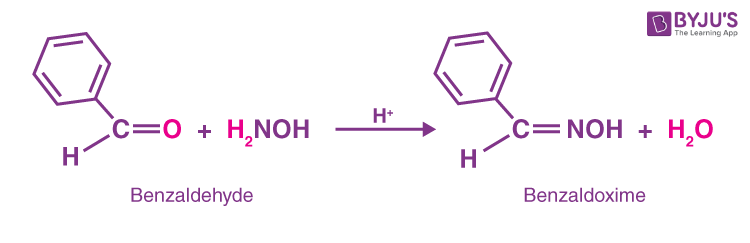
Oxides are more acidic than hydroxylamine `(NH_2 OH)`. Conjugate base of oxime is resonance stabilised. - Sarthaks eConnect | Largest Online Education Community

An Integrated Process for the Synthesis of Solid Hydroxylamine Salt with Ammonia and Hydrogen Peroxide as Raw Materials | Industrial & Engineering Chemistry Research

Formation of Aromatic Amidoximes with Hydroxylamine using Microreactor Technology | Organic Process Research & Development
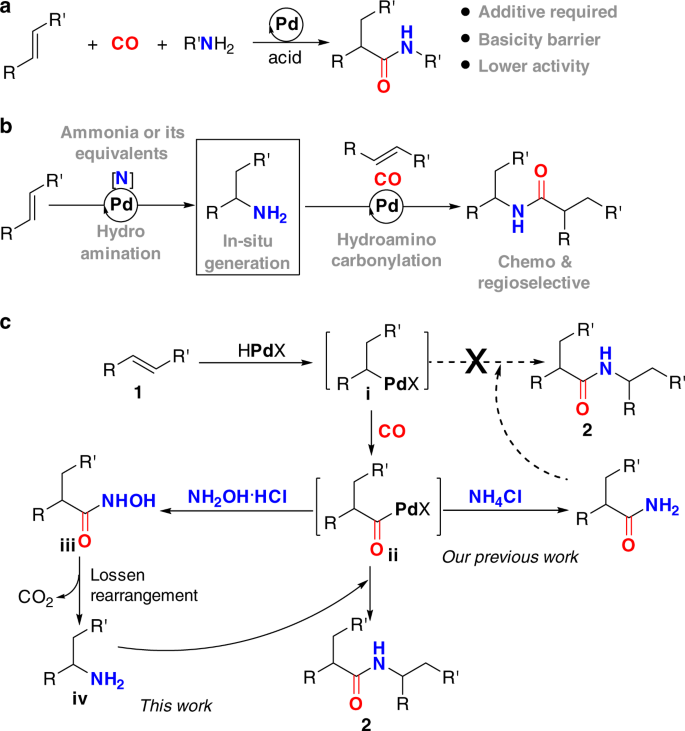
Palladium-catalyzed relay hydroaminocarbonylation of alkenes with hydroxylamine hydrochloride as an ammonia equivalent | Communications Chemistry

Hydroxylamine is a chemical mutagen that causes exclusively C to T transition mutations. A) Draw a hypothetical flow chart-using your own DNA sequences-to illustrate how a mutation can become incorporated into the
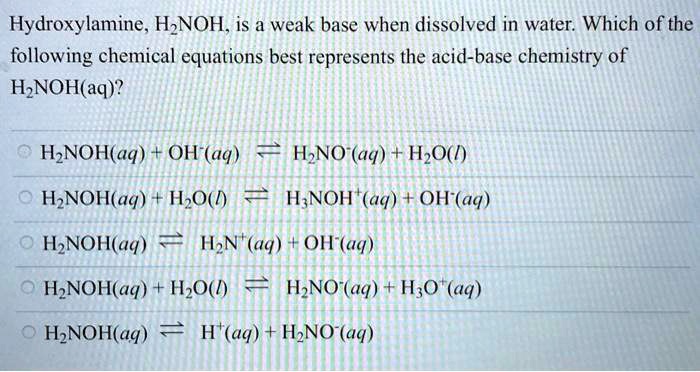
SOLVED: Hydroxylamine, HNOH, is a weak base when dissolved in water. Which of the following chemical equations best represents the acid-base chemistry of HNOH(aq)? a) HNOH(aq) + OH-(aq) b) HNO(aq) + H2O(l)

Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com
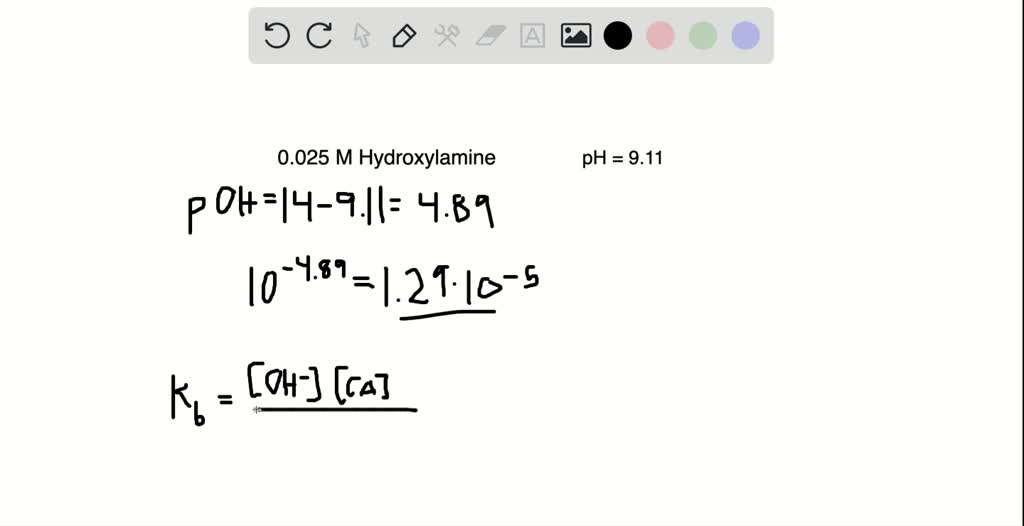
SOLVED:A 0.025 M solution of hydroxylamine has a pH of 9.11 What is the value of Kb for this weak base? H2 NOH(aq)+H2 O(ℓ) ⇄H3 NOH^+(aq)+OH^-(aq).