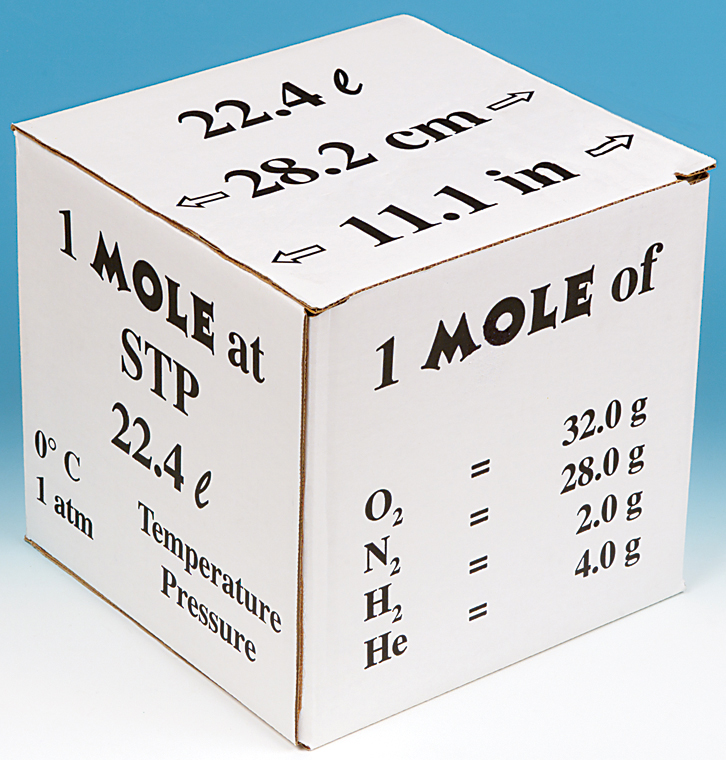
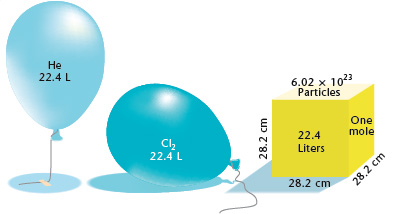
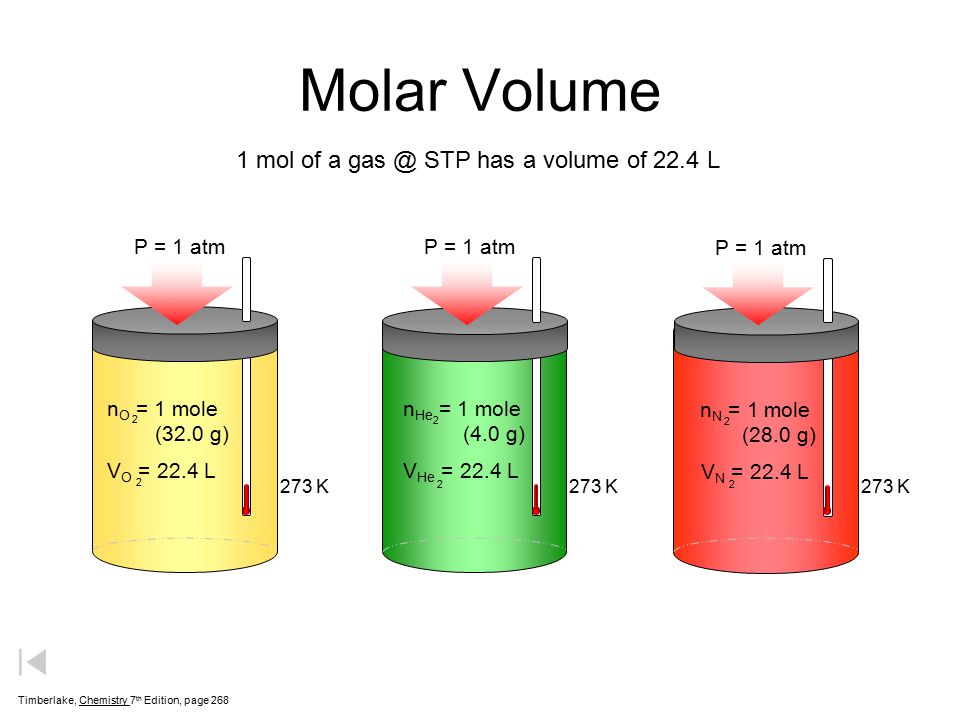
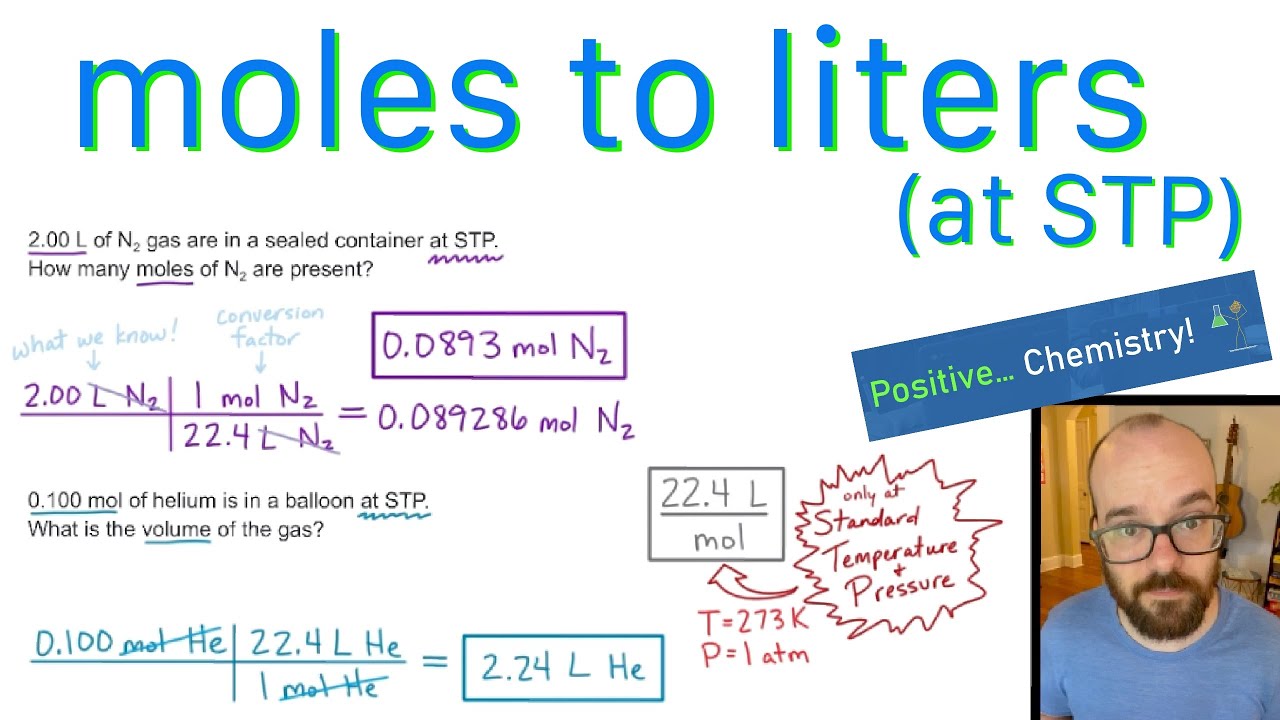
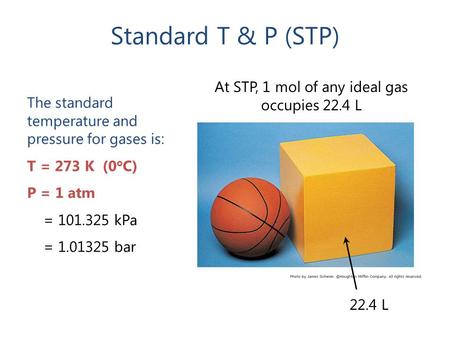
Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download

SOLVED:Calculate One mole of a gas occupies a volume of 22.4 L at STP. Calculate the temperature and pressure conditions needed to fit 2 mol of a gas into a volume of
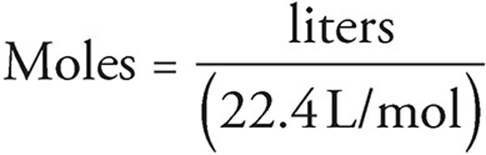
MOLES - Atoms, Elements, and the Building Blocks of Matter - Content Review for the AP Chemistry Exam - Cracking the AP Chemistry Exam